What Is A Physical And Chemical Change
Whatsoever affair can undergo two types of changes: Concrete or Chemic changes. The physical changes are those actions that generate no new substance. Instead, the older substance just changes in its size, structure and sometimes texture. Whereas the chemical changes give rise to a very new substance. And the older i loses all its feature properties.
The physical changes are reversible as the original affair can be brought back together afterward proper treatment. They are a temporary type of alter. While in comparison, the chemical changes are irreversible every bit the reactant losses its originality. Besides, these changes are permanent ones.
The concrete changes exercise not hamper the chemical composition of the substance. And at that place is no making or breaking of bonds. Whereas, in chemical changes, the chemical limerick of the matter is also changed. This is because the continuous making and breaking of bonds occur here.
This section volition discuss the differences between physical and chemic change.
Content: Concrete Vs Chemical Change
- Comparison Chart
- What are Physical Changes?
- What are Chemical Changes?
- Key Differences
- Conclusion
Comparison Nautical chart
| Basis For Comparison | Concrete Modify | Chemic Change |
|---|---|---|
| Significant | Physical changes are those changes, where there is no change in the mass of the substance and fifty-fifty the internal properties of the molecules remain same. Such changes are temporary. These changes involves the transformation in unlike phases of matter like from solid to liquid; liquid to gas or vice versa. | Chemical changes are those changes, where there is the formation of the new substance and such changes are permanent. There are the breaking and formation of new bonds to make the new compounds. |
| Information technology affects | It affects merely physical properties of the material like size, color and shape, etc. | It affects physical equally well equally the chemic properties of the material. |
| Produces | No new substance is formed, that means the molecules and atoms are bundled in the same way in products equally were in reactants. | Chemical changes always produce new substances, the atoms and molecules rearrange themselves and form a new compound. |
| Energy | In that location is no free energy production during physical changes. | The free energy is always required or produced in the form of lite, heat or audio during the chemical changes. |
| Reversible/Irreversible | Concrete changes are by and large reversible. | Chemic changes are irreversible or some time reversible also. |
| Examples | 1. Changing of ice into water or vice versa. 2. Tearing of paper. 3. Shaping the clay in unlike shapes. iv. Cutting a wooden pile. 5. Switching on/off electric equipment. | 1. Digestion of food. 2. Burning of a matchstick, fuels. 3. Cooked or riped vegetables, fruits. four. Getting old. v. Germination of curd. |
What are Concrete Changes?
The physical changes are responsible for transforming the outer advent of whatever substance. This can be related to the color, construction, shape, size, volume etc. But these changes never disrupt the molecular makeup of the given matter. There are no alterations in the chemic properties, thereby are easily reversible.
Definition of Physical Change
We can define concrete changes as:
"The process that changes in the external physical features of the substances leading to the alter in shape, size, texture etc., is a concrete alter".
Characteristics of Physical Change
- The concrete changes are reversible in nature. We can regain the original fabric after some physical processes.
- These changes don't generate any sort of energy during the grade of a reaction.
- Since the final production can easily exist transformed back into the original substance. Thus, they are temporary changes.
- The initial substance doesn't lose its substantial or characteristic identity.
- There is no change in the substance'south molecular configuration during the concrete reactions.
- The molecules might rearrange themselves by increasing the intermolecular spaces. Just the chemic composition of the molecules is never altered.
- The significant reason for the above is the absence of bond breaking or making during the physical changes.
- These changes requite rise to no new substances. Instead, the original product changes either in size, shape, structure or texture.
- The physical reaction might not release energy, but they need to absorb the energy to carry out the process in some cases.
- These changes are fast to occur, just they tend to last for a very shorter span of fourth dimension.
What happens during a Physical Change?
During the physical changes, the physicality or the external structure modifies. But the matter's uniqueness and chemic makeup remain unchanged.
For example, when the h2o (liquid) transforms into h2o vapour (gas). Or when ice (solid) melts into water (liquid), in every grade, the chemic composition remains the same, i.e., H2O.
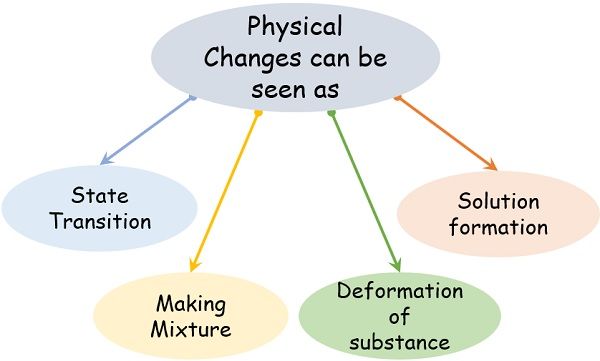
At the time of physical changes, we tin observe the following concrete transformation processes:
- State transition
- Deformation of the affair
- Generation of a mixture
- Formation of solution
Examples of the Physical Changes
1. Humid H2o: The water from its liquid state changes into vapours i.e., its gaseous country. Here, the chemical composition of water remains the same. Just the land transition occurs.

2. Crushing the Tin: On applying force the structure of the tin gets disrupted. But still, yous can easily identify it as a tin can as there is no major chemical alter. You can opposite dorsum the original structure of the can.

three. Melting of Ice: The water ice is a solid form of water which when melts gets converted to h2o. Here also, the state transition occurs.

iv. Germination of Mixture: Mixing two or more substances generates a mixture. Every bit the affair here doesn't undergo whatsoever chemical or molecular interaction. Thus, information technology is a concrete type modify.

5. Cutting Newspaper Strips: When the newspaper is cut fragmented into smaller strips simply never losses its uniqueness or chemical structure. Therefore, it also is a physical change.

What are Chemic Reactions?
We often refer to these changes equally a chemical reaction. Chemical changes are those which alter the chemic composition of the initial substance. These changes event in the rearrangement of atoms and molecules present in the original material. Thereby they generate newer substances with totally different properties than their parent compound.
The components that we take at the get-go of the reaction are reactants. These reactants serve the typical office of ingredients for any given recipe. Later, as the reaction terminates, the stop result is the production of the reaction.
The reactant molecules constantly make and suspension the chemic bonds for this conversion. Equally a effect, either some amount of the energy volition exist absorbed, or the energy will be liberated.
Definition of Chemical Changes
Nosotros can ascertain the chemic changes as
"The process that changes both structural and molecular properties of the reactant either due to making or breaking of the chemical bond, thereby developing a new product".
Or
"An irreversible or permanent change alters the molecular configuration of the substance to requite rising to the product".
Characteristics of Chemical Change
- During the chemical changes, the energy evolves or go absorbed either in the form of heat or light.
- These changes are irreversible in nature as the initial reactant losses all its properties. And thus, cannot exist regained easily.
- As we cannot transform the product back into the reactant, they are considered to be permanent changes.
- Completion of chemical reactions produces a new substance or substances with new properties.
- These changes take fourth dimension to occur and tend to last for a longer span.
- Chemical changes transform both the chemic and the concrete properties of the reactants.
What happens during the chemic alter?
During the chemic modify, the process of breakdown and formation of the bonds constantly occurs. This leads to the alteration in the free energy of the reaction. This change in energy facilitates the formation of a new substance with inverse features. Such every bit contradistinct melting signal, humid signal, color, texture etc.
For case, burning the thin ribbon of magnesium generates white powdered ash of magnesium oxide. Further, if we mix this ash with h2o, it will produce an aqueous solution of magnesium hydroxide. All the components hither accept different structural and chemical backdrop.
Along with the production, the chemic changes tin likewise lead to:
- Emission of oestrus, light or radiation
- Production of sound
- Change of odour or generation of any other significant scent
- Germination of bubbling
- Development of gas
- Change in colour of the reaction mix
- Fluctuation in the temperature
Examples of Chemical Change
ane. Baking of Cake: Blistering provides rut energy to the molecules. On heating, the molecules become excited for the formation or breakdown of the bonds. Thus, a new production is generated.

2. Burning of Woods: When we burn the woods, it is a combustion reaction. It chemically alters the woods into ash and generates several other gases like carbon dioxide, carbon monoxide as byproducts.

three. Cooking Eggs: The eggs are all proteins and fats. As the temperature rises during the cooking process, the egg proteins start getting denatured. Thereby changing the molecular orientation and chemic composition of the molecules.

4. Lightening Candle: Burning a candle too converts the wax into several other compounds thereby irresolute its chemical backdrop. Thus, it is a chemical change.

5. Curdling of Milk: During the curdling, the milk proteins coagulate. This changes their chemical composition. And the milk produces a completely new production i.e., Curd; having dissimilar backdrop than its parent chemical compound.

Key Differences Between Concrete and Chemical Changes
- In Physical changes, the fabric the mass of the substance and the internal organization of the molecules and atoms remains the same. On the contrary, when changes involve the germination of the new substance from the original substance it is a chemic change.
- Physical changes are temporary and they involve the transformation in different phases of matter. Similar from solid to liquid; liquid to gas.
Whereas chemical changes are permanent as information technology participates in the breaking and formation of new bonds to make the new compounds. - The physical alter affects only the physical properties of the material like shape, size, colour. Whereas chemical backdrop touch on not every bit physical as well as the chemical backdrop of the fabric. This is because there is an internal change in the atoms and molecules of the fabric.
- No new substance is formed in concrete changes while in chemical changes always produce new substances. , the atoms and molecules rearrange themselves and form a new compound, and these tin can be irreversible or reversible.
- There is no energy required or produced during physical changes, but in the chemical alter, there is always the requirement of energy. The energy is either absorbed or released in the grade of light, rut or sound.
Conclusion
From the to a higher place article, we tried to focus on the points on which the physical and chemical changes differ. As these are some of the mutual things that occur every then and now and i should know of this. Nosotros as well provided a few common instances which will be helpful for the readers to sympathize.
Source: https://biodifferences.com/difference-between-physical-and-chemical-change.html
Posted by: hardydocketook.blogspot.com


0 Response to "What Is A Physical And Chemical Change"
Post a Comment